Amines Are Used To Produce Dyes, Rubber, Pharmaceuticals, And Synthetic Resins And Fibers
 |
| Amines |
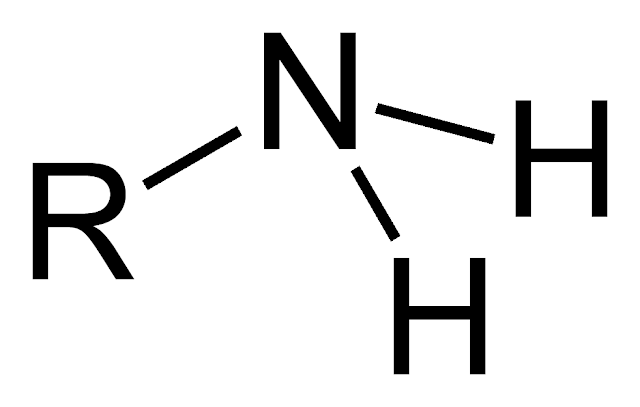
Amines are organic compounds that
have a functional group consisting of a nitrogen atom with a lone pair of
electrons attached to at least one organic hydrocarbon group. The organic group
can be a single alkyl, aryl, or aromatic ring. They are often called by their
IUPAC name, which is a combination of the prefix “amino-” and the suffix
“-amine.”
Amine molecules have high melting and
boiling points. This is because the lone pair of electrons on the nitrogen atom
can form hydrogen bonds with other molecules. These are very strong
interactions and require a lot of energy to overcome.
Aside from their high melting and
boiling points, they have several other interesting physical properties. For
example, they have a trigonal pyramidal shape with nitrogen at the apex. Amines also have bond angles of 107 deg. This
is the highest degree of symmetry that can be achieved in an organic compound.
They can act as both weak
Bronsted-Lowry bases and Lewis bases. Because of the lone pair of electrons on
nitrogen, they can easily form bonds with other molecules.
They can be used to neutralize acids
in various products and processes. In addition, they can be reacted further to
form derivatives with unique functionality for a variety of applications.
Chemical reduction and reactions of
ammonia with organic compounds are the major methods for producing these
compounds in industrial applications. A number of reactions can be carried out
with these compound, including the substitution of oxygen with a hydrogen atom,
as well as the alkylation of alcohols or other acyl groups by ammonia. Ethyl
and benzeneamines are two examples of industrially manufactured. These are
commonly used in making rubber, dyes, pharmaceuticals, and synthetic resins and
fibers.
Aniline and ethanolamines are also
widely used in water treatment applications. Amines are long-chain fatty compounds that lay a microscopic film
on the surface of the water and thus help to prevent fouling by removing dirt,
metals, and other foreign particles from the water.



Comments
Post a Comment